Definition
A polymer is a long repeating chain or three-dimensional network of smaller molecules called monomers. Polymers have lower densities and structural strength compared to ceramic or metals but are very ductile and pliable making them efficient for complex shapes or flexible structures (Daniel E. Heath, 2012). This also means they can be easily reformed, reused, and recycled (i.e. although this is may not apply to thermoset polymers. Typically, when naming polymers, one could add poly- in front of the name of the monomer utilized in polymerization. For example, ethene is a simple and very common monomer. As a polymer, this monomer now becomes polyethylene. Polymers created with only one species of monomer are referred to as homopolymers, while polymers created with two species of monomers are known as copolymers.
A polymer is formed through a process called polymerization in which a monomer is bound to an identical monomer in a repeating fashion. During Polymerization, double bonds are broken down in monomers in order to form sigma bonds to allow a monomer to bind with identical monomers on both sides completing the chain. Sigma bond is a type of covalent bond that takes place in the bonding process during polymerization.
Types of Polymerization
Polymerization can either be classified as addition or condensation polymerization. Addition Polymerization refers to the Process in which monomers are added to each other in a sequence and no byproducts are formed. The steps of addition polymerization are initiation, propagation, and termination. In order for Addition Polymerization to be initiated a free radical must be introduced. Free radical refers to atoms or ions that have a single unpaired electron (Highly Unstable). During polymerization, the free Radical separates the double bond found in monomers and binds to one of the carbon atoms. This resulting reaction causes an odd number of paired electrons meaning that one of the carbon atoms now has an unpaired electron and has become a Free Radical. This creates a process in which the unstable monomer will keep bonding to other molecules/atoms in order to satisfy the Free Radical. Termination refers to the end of the process in which another free radical is introduced in order to satisfy the process ending the cycle to eliminate all Free Radicals ending Polymerization.
Condensation polymerization refers to the process in which two molecules join and water is formed as a byproduct. This is commonly found in natural polymers. The production of nylon is a perfect example of a polymer formed with condensation polymerization. Nylon is created as a result of the combination of Hexamethylene diamine and adipic acid. Hexamethylene diamine contains an amine group and adipic acid contains a carboxylic acid. When Nylon is formed the left-over hydrogen and oxygen molecules form water as a byproduct.
Copolymerization simply refers to the process in which copolymers are formed. They can also be addition or condensation.
Manipulating Polymerization
Cross-linking is the process in which polymers are bonded to each other in order to form a network polymer. The network polymer will be a three-dimensional polymer with multiple branches. This can be done in order to reinforce the polymer as well as make it more durable. Vulcanization is the process of cross-linking in which Sulfur is used to assist in the procedure and strengthen Elastomers and rubbers. On heating, linear polymers flow and can be considered thermoplastic. To prevent flow, polymers are sometimes cross-linked in order to make them thermoset. Cross-linking bonds the chains together to form a network. The resulting product is called a thermoset, because it does not flow on heating and therefore cannot be recycled (Sperling, 2006).
The process of altering the elements or substituents of a monomer to change its properties is often utilized in synthetic polymers in order to produce desirable traits. For example, changing one Hydrogen in an ethylene monomer for a methyl group results in the propylene monomer.
-
Methyl group refers to a group of atoms comprised of three hydrogen atoms bonded to a carbon atom.
When changing one Hydrogen in an ethylene monomer for a Phenyl group the resultant monomer is styrene.
-
Phenyl groups have six carbon atoms bonded together with five of which are bonded to individual hydrogen atoms.
Styrene was given its name due to the manipulation first being conducted from a tree in the styrax family the resulting monomer was named Styrene (Course, 2014). Now because of this, you can probably realize that Styrofoam was given its name due to Styrene being transformed into a foam.
When changing all Hydrogen in an ethylene monomer for Fluorine it can be renamed to Tetrafluoroethylene. This polymer is commonly used in lubricant applications due to the properties of flourine. Fluorine is an extremely electronegative element meaning it is highly attracted to itself but cannot bond with atoms of different elements efficiently. Electrons in this monomer are not available for interaction with any other molecule or atoms. This makes Polytetrafluoroethylene (PTFE) or Teflon common in applications in lubrication and non-stick products.
Polymer Properties
The molecular properties of a polymer can be used to determine and characterize:
-
The temperature in which a polymer that is initially glassy begins to transform into a rubbery state
-
This is known as (Glass Transition Temperature)
-
For example, cooked ramen noodles are an example of a polymer network in the rubbery state
-
Before they are cooked they are stiff and brittle representative of a glassy polymer
-
The networks are still interlinked but, and each polymer chain placement is relative to its neighboring polymer chain
-
When they are cooked they lose their rigidity and become flexible and are able to flow somewhat freely
-
-
-

The Rubbery State
-
Rubber polymers are amorphous
-
This means in the solid-state the arrangement of the atoms is random
-
-
They are soft, flexible, and extensible
-
Elastomers can be classified as existing in the rubbery state
-
Due to cross-links in some amorphous polymers, they do not melt or become a liquid when heated up (also referred to as thermoset)

The Glassy State
-
As the temperature cools the molecular movement also drops causing the movement of the polymer chain to become slower and slower causing the material to be stiffer and stiffer
-
Eventually, at a certain point, the temperature will drop to a point in which the random coils will have frozen in place
-
Once this has occurred the polymer has entered the glassy state
-
The temperature in which this occurs is known as Tg (Glass Transition Temperature)
-
-
Molecules in the glassy state can no longer rearrange themselves
-
This means the material will become brittle, meaning it has very low ductility and toughness
-
-
An example of the glassy state would be uncooked ramen noodles in a package
-
All polymers form glasses at low enough temperatures

Course, C. (2014, January 6). Polymers: Crash Course Chemistry #45. Retrieved November 18, 2020, from Polymers: Crash Course Chemistry #45
Polymer Backbone
The backbone of a polymer is essentially the line that makes up the majority of the polymer.
-
Ethylene monomers have a carbon backbone, with pendant hydrogen groups attached
If a polymer chain is made up of only one type of repeating monomer, it is referred to as a homopolymer and if it contains different subunits, it’s called a copolymer.
Polymer Chain Length
As the chain length increases, melting and boiling temperatures increase, viscosity is increased and mobility decreases.
Potential Molecular Structures of Polymers
-
Linear Polymer
-
A linear polymer is a polymer comprised of monomers arranged in a straight line
-
A linear polymer consists of a single continuous chain of repeat units
-
This chain is called the backbone of the polymer
-
-
-
Branched Polymer
-
Occurs when intended or unintended reactions occur during polymerization and the products bind to the backbone of the polymer
-
Can also be formed when two linear polymer molecules are covalently bonded together
-
-
Network Polymer
-
Occurs when multiple branched polymers are linked together
-
Polymer representation in Stress-Strain Plot
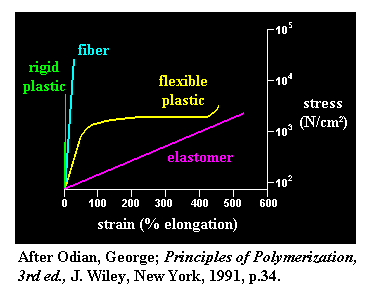
As you can see in the plot the elastomer has a linear and constant relationship between stress and strain due to its rubber-band-like nature. Although a linear plot does not always signify an elastic material. The rigid plastic also has a linear relationship but this is because the material itself does not undergo plastic deformation and will likely fail before deforming plastically. Similarly, glass or ceramic could potentially have a similar plot as rigid plastic due to its low resilience.
References
-
Baheti, D. P. (n.d.). British Plastics Federation. Retrieved from How Is Plastic Made? A Simple Step-By-Step Explanation: https://www.bpf.co.uk/plastipedia/how-is-plastic-made.aspx#[3%20NEW]
-
Daniel E. Heath, S. L. (2012). Chapter 1.2.2 Polymer: Basic Principles. In A. S. Buddy D. Ratner, Biomaterials Science; An Introduction to Materials in Medicine (pp. 64-82). Academic Press.
-
Madhu. (2018, July 12). DifferenceBetween.com. Retrieved from Difference Between Organic and Inorganic Polymers: https://www.differencebetween.com/difference-between-organic-and-inorganic-polymers/#:~:text=The%20key%20difference%20between%20organic%20and%20inorganic%20polymers,do%20not%20contain%20carbon%20atoms%20in%20the%20backbone.
-
Matthew Treiswe, S. A. (n.d.). Degradable and Resorbable Biomaterials. In A. S. Buddy D. Ratner, Biomaterials Science (pp. 179-195).
-
R. Chandra, R. R. (1998). BIODEGRADABLE POLYMERS. Dehli: Pergamon Press.
-
Reusch, W. (2013, 5 5). Chemistry.MSU. Retrieved from Polymers: https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/polymers.htm
-
Sperling, L. H. (2006). Introduction to Physical Polymer Science. John Wiley & Sons.
